Yellow Diamonds are the most common among all fancy colored diamonds. Yet, paradoxically, orange diamonds, their closest chemical siblings, are among the rarest gemstones.
74.48-carat Fancy Vivid Yellow Internally Flawless Diamond
These diamonds are the best way to introduce people to the world of colored diamonds because they are relatively plentiful. In fact, for many people, a yellow diamond may be the only colored diamond they ever see in a jewelry store.
Natural fancy yellow diamonds should not be confused with transparent (or white) diamonds of inferior quality, which also appear pale yellow due to trace nitrogen impurities. The color grades of white (transparent) diamonds range from D to Z. Grade D diamonds are the whitest/clearest and most valuable. In contrast, grade Z is tinted with faint sickly yellow to brown and is the least valuable in comparison.
When Does A Yellow Diamond Enter The Fancy Yellow Club?
However, a stronger yellow beyond the Z color grade confers the coveted fancy yellow grade upon the diamond. Such a diamond is highly sought after by celebrities, royalties, fashion-forward, and jewelry designers. The diamond becomes pretty expensive and goes beyond the reach of most of us.
Click On The Images
As the diamonds become progressively darker yellow in color, they are given their own separate color grades: fancy light yellow, fancy yellow, fancy intense yellow, and fancy vivid yellow (also known as canary yellow).
 |
 |
 |
| Fancy Vivid Yellow Diamond | ||
 |
 |
 |
| Fancy Intense Yellow | Fancy Intense Yellow | Fancy Yellow |
 |
 |
 |
| Fancy Yellow | Fancy Yellow | Fancy Light Yellow |
 |
 |
 |
| Fancy Light Yellow | Y-Z Yellow | Y-Z Yellow |
 |
 |
 |
| W-X Yellow | W-X Yellow | U-V Yellow |
 |
 |
 |
| U-V Yellow Diamond | S-T Faint Yellow Diamond | G-color-white-diamond |
Nitrogen is the Most Rampant Impurity in a Yellow Diamond Crystal Lattice:
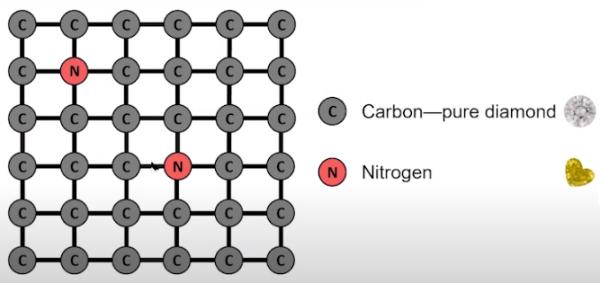
Nitrogen impurities absorbed in a diamond’s crystal lattice while growing deep inside the earth primarily impart color to yellow and orange diamonds. The most prevalent impurity in natural diamonds is Nitrogen. Two reasons make this gaseous element so pervasive.
Firstly, Nitrogen is a superabundant element accounting for more than seventy-five percent of the earth’s atmosphere. Whatsmore it is the seventh most abundant element in our universe.
Secondly, carbon and Nitrogen closely match atomic radii of 170 and 155 picometer (Van der Waals radii) respectively. Consequently, the crystal framework of the diamond inexorably assimilates Nitrogen in its vicinity.
Virtually all yellow and orange diamonds acquire their color from four core defect groups. They are:
1) Cape defects:
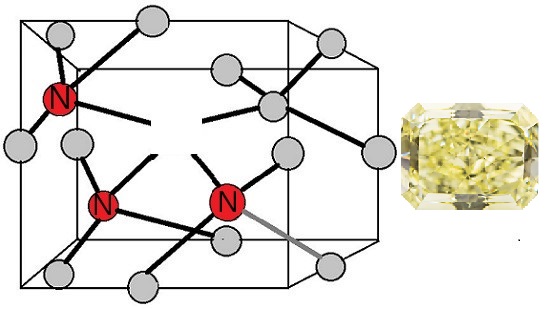
Gemologists first used the word “cape” to describe yellow stones that were pale to deep yellow from South Africa’s former Cape Province. The region was famed for its yellow diamonds. The term now refers to diamond from any geographical region that is usually light yellow and has a color from the N3 group.
N3 Cape Defect in a Diamond
Most diamonds in the GIA color grading scale’s D-to-Z range acquire a bit yellow because of the cape defects. On the other hand, plastic deformation rather than nitrogen impurities cause brownish tints in some D-to-Z diamonds.
Gemologists think cape defects are poor light absorbers, meaning a diamond needs them in copious quantities to display a saturated yellow color. Moreover, the light should trace a longer path, like in diamonds cut with larger facets.
Figure 1: Cape Defect and C-centre Color Comparison
For example, among the transperent diamonds, an N-color diamond (lower on the GIA color scale) shows more yellow than a G-color diamond of similar cut proportions and size. The added yellow is because it has appreciably higher cape defects.
In the same way, there will be still more defects in a fancy yellow cape diamond of the same size and cut.
Click On The Images
2) Isolated nitrogen defects:
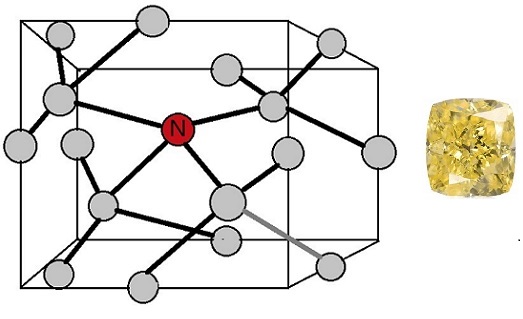
The second most prevalent cause of color in natural yellow diamonds is single atoms of Nitrogen replacing carbon in the diamond lattice. They make up just over thirteen percent of the stones GIA analyzed.
Isolated Nitrogen C-center Defect
Compared to cape-defects, C-centers are more powerful light absorbers—concentrations of just a few parts per million isolated nitrogen atoms yield an intense yellow color. Consequently, connoisseurs call these diamonds “true canaries” and admire them greatly.
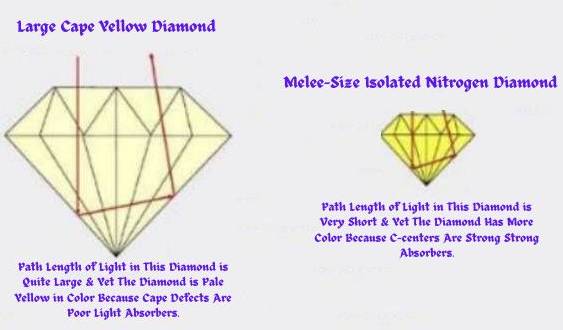
This study examined diamonds larger than melee sizes. Nevertheless, C-centers color almost all intense color grade melee-sized natural diamonds. This is because the length of the light absorption path is very short in melee diamonds. Therefore, only the strong light-absorbing C-centers can impart the yellow color (see figure 6).
Natural diamonds are hardly ever able to retain the C-centers. The eons of the geologic time demolish any existing C-centers by aggregating them into A-centers. In contrast to C-centers that induce intense yellow, the A-centers do not produce any color. Deeper yellow colors arise out of more significant concentrations of C-centers Figure 6.
The proportion of absorbed and propagated light controls the quantum of imparted color. Furthermore, light-absorbance is an outcome of the length of the light path, the defects’ saturation, and the defects’ absorption efficiency.
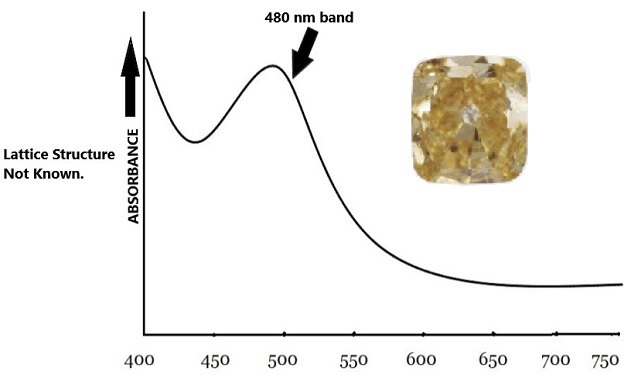
3) The 480 nm visible absorption band:
A broad absorption band centered at 480 nm in the visible spectrum is one of the more intriguing ways diamond obtains its yellow color.
480nm band
Gemologists still do not wholly comprehend the configuration of the 480 nm band. However, meticulous analysis of the phenomenon shows that it is almost always linked to both A-centers (nitrogen pairs) blended with a smattering of C-centers. Some researchers say that substitutional oxygen atoms in the diamond lattice cause this defect—the 480 nm band colors around five percent of all yellow diamonds.
The Gemological Institute of America has had the good fortune of analyzing hundreds of thousands of diamonds with yellow or orange hues in the past decade. The unprecedented data quantum helped them conclude that two-thirds of these diamonds had pure, unmodified yellow hues. On the other hand, not more than one-tenth of a percent of the stones examined over the same period had pure, unmodified orange hues.
Interestingly, diamonds colored by the 480 nm absorption-band show color change with temperature change. For example, when heated up to 500 degrees celsius, such a diamond shifts its color towards an orange hue.
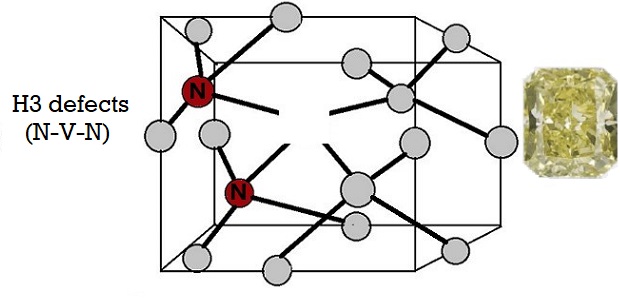
4) H3 defects:
H3 Lattice Defect in a Diamond
H3 is an uncharged defect of two nitrogen atoms next to a vacancy in the diamond lattice. It has a zero-phonon line (ZPL) at 503.2 nm. H3 absorption creates yellow color on its own, and it does so in about 2% of yellow diamonds.
Role Of Nitrogen Atoms:
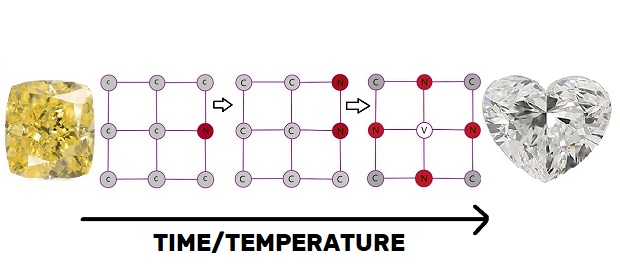
Scientists have established that in the early stages of a diamond’s growth, isolated nitrogen atoms (not clusters) diffuse evenly into its crystal lattice by swapping carbon atoms.
Consequently, all nitrogen-carrying natural diamonds start as bright yellow (canary) or orange ‘type 1b’ diamonds.
High temperatures and pressures push the nitrogen atoms into clusters as the diamond grows. Consequently, the gems turn into near-colorless stones or yellow diamonds colored by ‘cape-defects.’
Diamond Type IB C-Centers Defects Aggregating Into TypeIaB (B-Centers)
Nitrogen is widespread in the earth, and diamond atoms are strongly attracted to nitrogen atoms. Consequently, Nitrogen in most diamond crystallization zones is easily assimilated into the diamond as it grows. How long nitrogen-containing diamonds spend at high temperatures deep in the earth usually determines whether they will be yellow.
Therefore, almost every mining region on the planet holds fancy-colored natural yellow & orange diamonds. But, be that as it may, specific sites are renowned for yielding fine fancy yellow diamonds of particular types.
Click On The Images
Some Specific Sites Reveal Particular Types of Yellow Diamonds:
The Cape region of South Africa gives us Type Ia cape diamonds, which is how they got their name. The Ellendale mine in Australia has also been a significant source of this type of yellow diamond.
Most diamonds stay deep in the earth, where it is hot, for long periods. As a result, type Ib yellow diamonds with C-centers are not very common. Nevertheless, some places, like the Zimmi deposit in Sierra Leone Ekati & Diavik mines in Canada, are known for having type Ib yellow diamonds that are surprisingly big.
Yellow & orange diamonds can both also be spawned in a laboratory or rendered by color treatments. As such, we should meticulously understand the defects behind the color of natural diamonds. This knowledge is crucial to differentiating between artificial and natural yellow color diamonds.
The volume of Nitrogen in the earth’s mantle is substantially lower than in its atmosphere. And yet, around ninety-eight percent of all mined natural diamonds (including colorless & fancy-color) stow measurable quantities of Nitrogen. Scientists use FTIR (Fourier Transform Infrared spectroscopy) technology to measure nitrogen content. Thus, we know that most places where diamonds grow are full of Nitrogen.
Click On The Images
Ways in Which Nitrogen Can Ensconce Itself Within a Yellow Diamond Crystal:
Moving further, let us try to visualize the many ways that Nitrogen can ensconce itself within a diamond crystal:
- Nitrogen can exist as evenly distributed single atoms substituting for carbon. They are called C-centers. Diamonds bearing this defect are called type Ib diamonds.
- Groups of Nitrogen atoms can occur in pairs. They are called A- centers. Diamonds carrying this defect are called type IaA.
- Atoms of Nitrogen can occur in clusters of fours. These are known as B-Centers, and stones embracing this type of defect are called type IaB.
- N3, H2, H3, H4, and NV are the More complex defects.
Diamonds with C-centers, N3, H3, and NV defects absorb light in the visible spectrum and thus acquire color. Diamonds with other types of defects (A- and B-centers) are devoid of color. However, they do absorb infrared light in a particular manner.
Click On The Images
Inference:
Fancy yellow and fancy orange diamonds are both opulent and exceptionally rare gems. But they don’t exist simply because nature adds Nitrogen to the diamond lattice. In addition, Nitrogen atoms need to satisfy two more conditions for the diamonds to acquire these colors.
Firstly, nitrogen atoms must be present in adequate quantity, and secondly, nature must configure them correctly to absorb light from the blue regions of the visible spectrum.
Furthermore, the same defects do not give rise to yellow and orange colors.
- The N3 defect and its associated absorptions collectively termed “cape” diamonds color most natural yellow diamonds.
- In addition, C-centers defects also color yellow diamonds.
- Still, other color centers like the 480 nm visible absorption band, H3, and H-related defects can sometimes add the yellow color to a diamond.
- On the other hand, the 480 nm visible absorption band or C-centers almost always give fancy orange diamonds their color.
- Orange color is also created occasionally as a primary hue in diamonds colored by the H3 defect in combination with a 550 nm visible absorption band or by nitrogen-vacancy defects.
Both yellow and orange diamonds share a markedly similar cause of color. Nitrogen-related crystalline defects induce both colors in diamonds. However, a subtle displacement in the visible spectrum’s transmission window contributes to the color change.
Diamond’s orange and yellow colors come from a blend of all the visible light above a specific wavelength exiting from the stone.
Most nitrogen-related absorptions in the blue region of the visible spectrum, below 510 nm, manifest the yellow color. On the other hand, absorptions extending to ~600 nm show us the orange color (figure 4A).
Furthermore, Different concentrations of the same defect can confer either yellow or orange to the diamond rough. For example, low concentrations of nitrogen defects can produce a pale yellow color.
Diamond Lattice With Nitrogen Impurity
Similar to white diamonds, the 4C’s color, cut, clarity and carat are the factors that need to be considered before choosing a yellow diamond. With Colored Diamonds, Clarity is not as important as Saturation of Color, and their prices depend on the saturation of color. A diamond must be certified by a gemological laboratory to be called natural.
Fancy Vivid Yellow Cushion Diamond Halo Split Shank Ring
Click On The Image
Or Learn About More Colored Diamonds As Follows:
See Also:
Fascinating Facts About Diamonds Straight From Tiffany & Co’s Chief Gemologist